Our Current Energy System
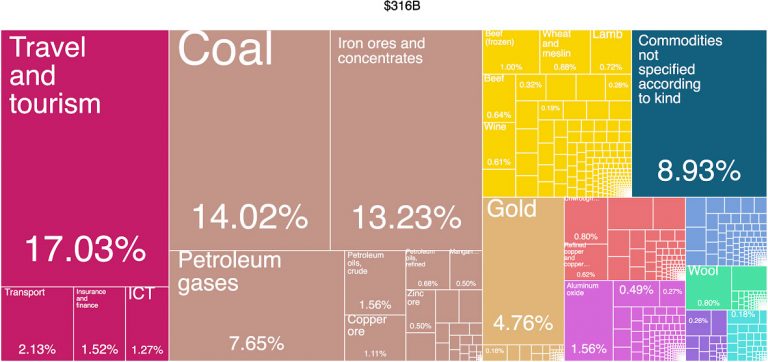
Currently, we rely on fossil fuels including coal, oil and natural gas to provide 87% of the world’s energy. Coal provides a great contribution to Australia’s economy and generates a large number of jobs – but for how much longer? Many countries are eager to shift from coal to cleaner energy technologies.
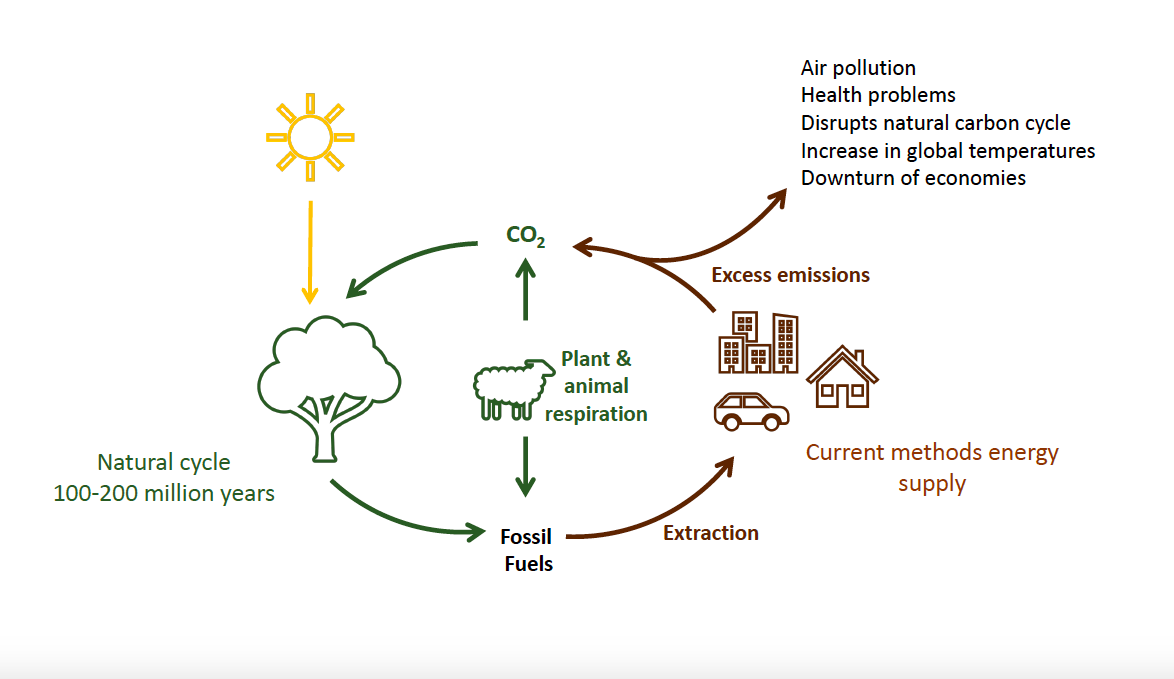
The earth has a natural carbon cycle, where carbon dioxide is produced naturally by processes such as decomposition, and then taken in by plants to grow and produce oxygen. Our current method of energy production involves extracting and burning fossil fuels, ultimately disrupting this cycle by generating excess carbon dioxide that cannot be converted back into fossil fuels (it usually takes a few million years). If the amount of CO2 we generate continues to increase, it is predicted that global temperatures will increase by at least 3 °C by the end of the century, which will change the earth’s ecosystem and have a huge impact on our daily life.
Why we need to shift from using fossil fuels as our energy source:
- Harmful gases such as carbon dioxide, carbon monoxide, sulfur oxide and nitrogen oxides are also released into the atmosphere from burning fossil fuels. This results in high levels of air pollution all over the world, which can lead to cancer and other health problems (World Health Organization).
- We are heavily reliant on fossil fuels for a variety of other applications, including plastics, pharmaceuticals and fertilizers to grow food for an ever-increasing population.
- Excess carbon dioxide in the atmosphere and oceans is leading to global warming. This is already having an impact on Australia’s climatic system, and in the future we will see more frequent bushfires, more severe/longer droughts and eventually some of our nation’s most beautiful landmarks such as the Great Barrier Reef will be gone.
Australia’s economy relies heavily on mining and exporting primary resources such as ores, coal and gas. What is going to drive the economy in the future and provide us with a healthy economy when these resources are gone?
What Could We Do Instead
Relying on energy resources that take millions of years to produce is not the most effective way to power our society. What if we could move towards an abundant yet sustainable energy system?
We have resources such as the sun, wind and water all around us, and with clever use of these resources we could have clean, affordable and infinite energy. Rather than fossil fuels being mined, households, businesses and industries could become completely self-sufficient, renewable, micro-energy systems. Australia could also become an international platform to harvest solar and wind energy, and export this renewable and clean energy to neighbouring countries.
The problem with this vision is the irregular nature of wind, solar and other renewables. Electricity production from renewables is seasonal, and any excess electricity produced must be stored for the days with no wind or less sun. However, there is currently a lack of energy storage systems that are more effective than batteries, making it difficult for Australia to become a global exporter in clean energy. The solution to this problem is to store additional electricity in the form of hydrogen because it is clean and very efficient. Hydrogen has an energy density of 39 kWh/kg, which means that 1 kg of hydrogen contains 130 times more energy than 1kg of batteries, meaning lots of energy can be stored with hydrogen in only a small volume.
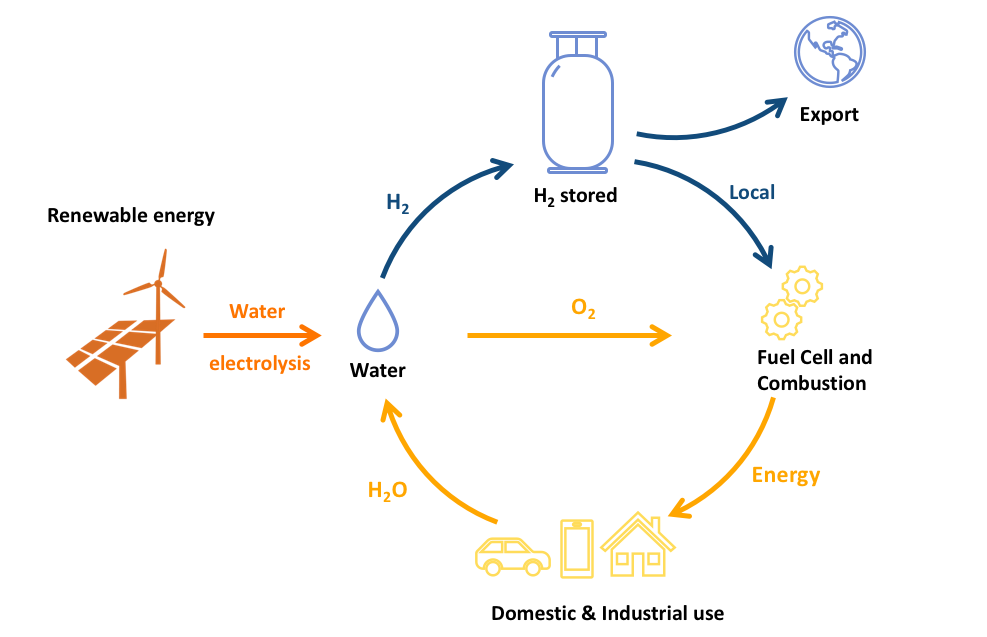
Solar panels, wind turbines or any other form of renewable energy could be used to generate electricity to locally power homes and domestic industries, and any excess electricity can be used for splitting water into hydrogen and oxygen (electrolysis), with the hydrogen stored for later use. The stored hydrogen can then be converted back into electricity or other forms of energy when needed, via a fuel cell or direct combustion. Using hydrogen this way provides a path for effectively harvesting renewable energy and a sustainable path for unlimited clean energy, with the only emission being pure water!
For large solar power plants in remote areas, electricity could be directly harvested, converted into hydrogen for storage and exported, making Australia a global leader in the clean energy market. In this new energy economy, hydrogen provides a very efficient means to store, transport and use energy on demand and in a clean fashion.
How Can We Make It Happen?
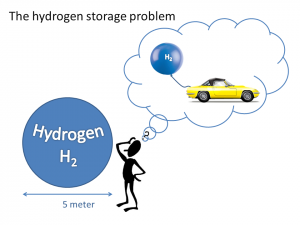
As a gas, hydrogen has a low density, i.e. it occupies a very large volume. We need to find ways to compact it into much smaller volumes for its practical and everyday use.
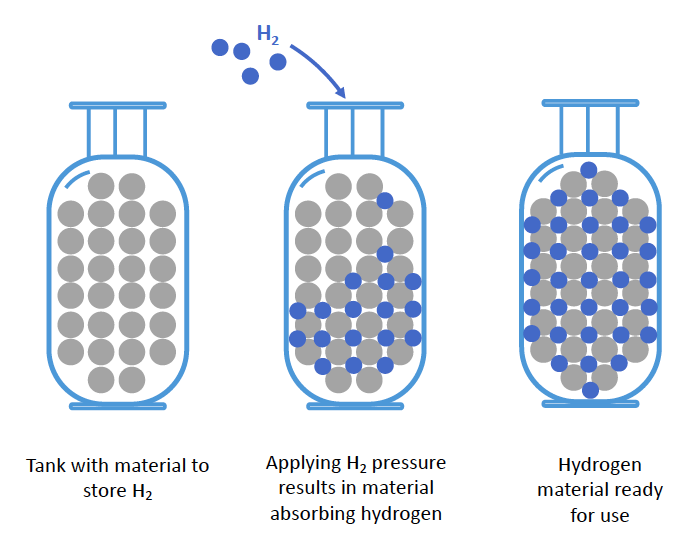
The solution is to use materials capable of storing large amounts of hydrogen in a compact form. Metals and compounds such as magnesium and sodium borohydride can absorb a lot of hydrogen (up to 10% of their own weight) like a sponge would absorb water. The beauty of this concept is that once the hydrogen is absorbed by the material it is indefinitely stored in a totally safe manner. Controlling the temperature of the materials will allow fully reversible uptake and release of hydrogen.
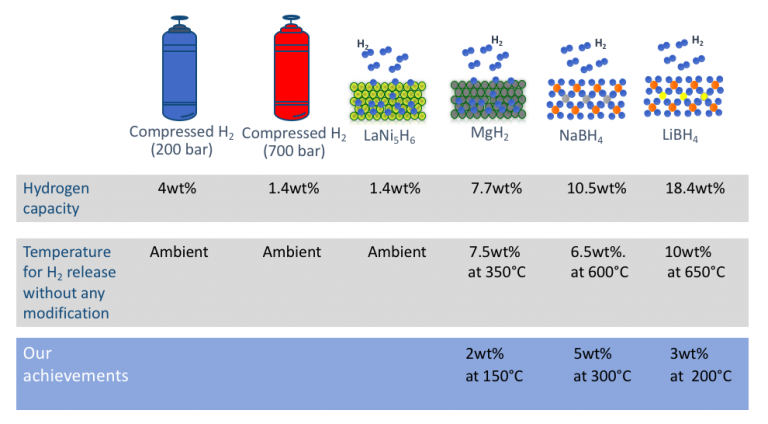
Today only a few materials (e.g LaNi5H6, see below diagram) can absorb and release hydrogen at ambient temperature. Unfortunately, these materials are heavy and thus can only store small amounts of hydrogen (less than 1.5 wt%, i.e. 1.5 % of their own weight).
Many other materials, like borohydrides, can store large amounts of hydrogen (up to 18.4 wt%). However, the use of this material is currently limited by the need for high temperatures to enable the release of hydrogen and extremely high pressures (above 300 times atmospheric pressure) for hydrogen uptake. We need to find a way to use these materials without the extreme conditions.
We have been doing R&D to bring temperature requirements below 100 °C and use low pressures so that practical hydrogen storage of H2 storage capacity > 5 wt.% can become a viable option. To be able to transform and extend our research findings into new market products we need your help and support to raise enough funds for this research to continue.
To learn more about donating to MERLin, please visit the Donations page.
Hydrogen FAQs
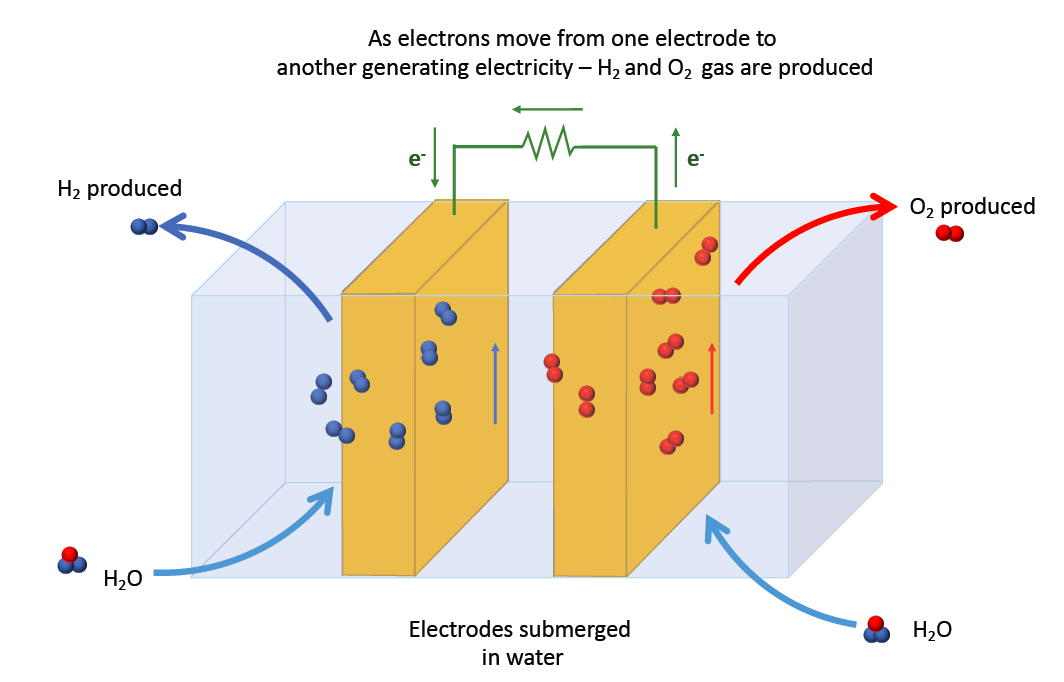
Hydrogen can be easily produced by passing electricity through water. This process known as electrolysis is a mature technology that can be easily implemented for small or large scale application.
For mobile applications, such as cars, the safety issue and accessibility of energy storage is important. Currently, hydrogen is generally stored in its liquid or compressed gas form, which makes it unsafe, and very difficult to refill the fuel. Therefore a cheap, safe and efficient way to store hydrogen is necessary for practical and everyday use.
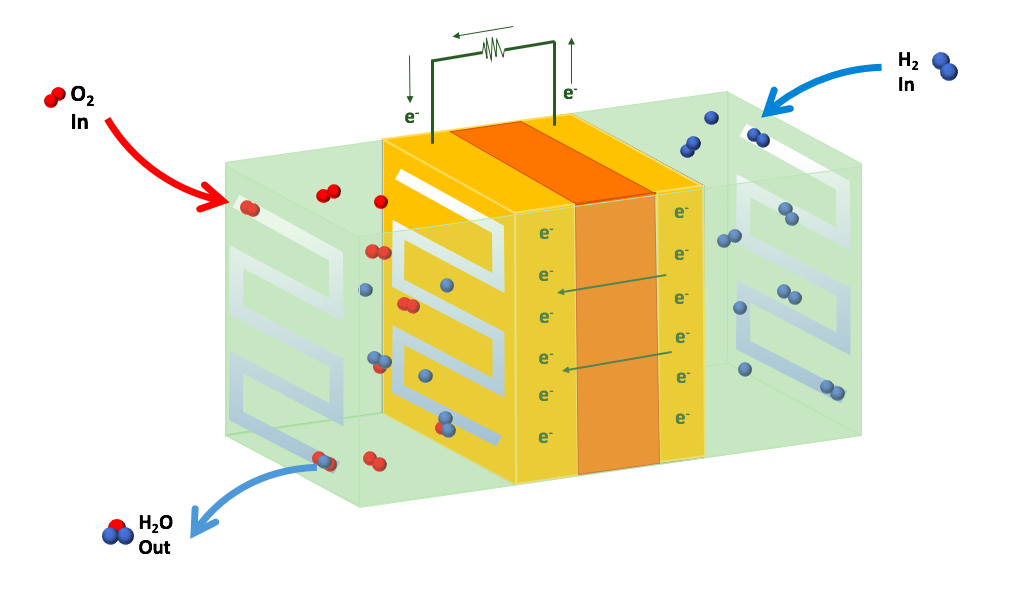
A fuel cell is a device that can react hydrogen and oxygen to generate electricity from chemical energy. It can continuously provide electricity if there is a constant supply of hydrogen and oxygen.
Hydrogen can be burnt in a fuel cell to produce electricity, or in a combustion engine, just like petrol, to be transformed into energy to move a car or produce electricity and/or heat. It has the highest density of any fuel (3 times that of gasoline). It is used to send people into space, all while producing zero harmful emissions! Hydrogen energy can be used for transport, powering homes and businesses as well as portable electronics – it truly has potential to be used for all our energy needs!
H2 and O2 are fed into the fuel cell, where they react with each other and produce water as a by-product. Electrons pass through the fuel cell generating an electric current.
Hydride materials absorb hydrogen like a sponge and are able to store very large amounts of hydrogen in a small space within their structure where hydrogen can be stored as an atom (H), or a hydride (H-), for example. Sodium borohydride (chemical formula NaBH4) is an example of a hydride – it consists of hydrogen bound to the boron (B) in the form of an anion (BH4- ) stabilised by sodium (Na+). Sodium borohydride because of its high hydrogen content can be used for solid-state hydrogen storage, which is what our current research focuses on in the aims of advancing better hydrogen storage technologies.
Currently, most hydrogen is produced from fossil fuels. The most common method is called ‘steam reforming of methane’, which involves natural gas reacting with steam to produce hydrogen, carbon dioxide and carbon monoxide. The problem with this is that it still produces harmful emissions including carbon dioxide (CO2). A way to avoid this is to use renewables solar and wind farms, for example, to produce electricity and split water (H2O) into hydrogen (H2) and oxygen (O2); a very clean process.
Materials like sodium borohydride can absorb hydrogen within their structure. Once the hydrogen is absorbed it is safely held within the material. A small amount of heat needs then to be applied to release the hydrogen. This makes the process of using hydrogen as an energy source extremely safe. The advantage of sodium borohydride is that it can hold a lot of hydrogen (i.e. 10.8 mass %).
Hydrogen is often incorrectly seen as an unsafe fuel; usually due to the 1937 Hindenburg disaster (it has since been proven that the fire was inconsistent to that of a hydrogen fire!). In fact, while hydrogen is flammable it is inherently no more dangerous than other conventional fuels such as gasoline or natural gas. Hydrogen is 14 times lighter than air, which means that even in the event of a leak it will rapidly diffuse into the air into a non-flammable concentration as illustrated below. Even if there is a leak, hydrogen is non-toxic and non-poisonous, and does not contaminate groundwater or air.
Hydrogen powered vehicle on the left, LZ 129 Hindenburg on the right.
3 seconds after ignition: Hydrogen escaping vehicle on right (equipped with a 200 bar compressed hydrogen tank) is burning as a clean flame. The temperature at the wheel in the hydrogen powered car did not exceed 40 °C. The driver can safely leave the vehicle.
What makes our technology even safer than compressed hydrogen is that the hydrogen is locked in solid materials: This is a well-established technology, and it is so reliable that hydrogen storage materials (called hydrides) are used as fire or overheat detectors in aircrafts.
The main reason we can’t is that renewables have problems with intermittency: During times of low sun or wind there is not enough power being produced to meet demand. But other times there is much more power generated than is required. Hydrogen provides a way to smooth out these peaks; it can be stored, and then released when there is a sudden cloud or the wind speed drops too low. Essentially hydrogen allows for continuous, reliable power that renewables alone cannot provide!
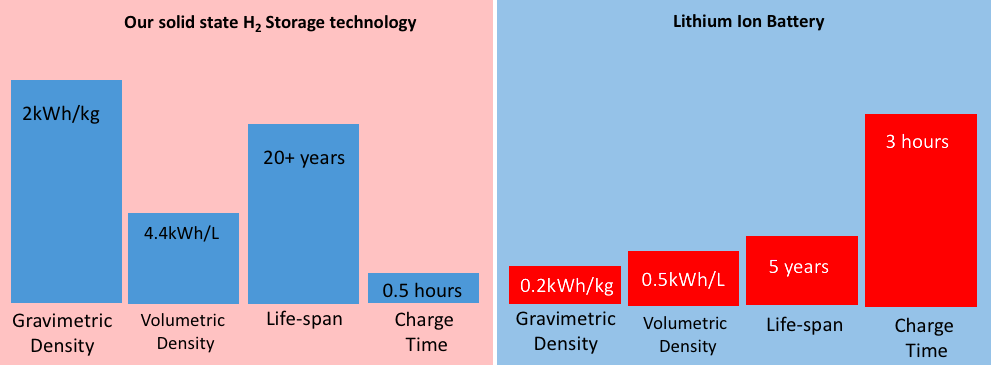
It is true that batteries serve much the same purpose as hydrogen storage, although there are a number of factors that make batteries a less effective storage medium compared to hydrogen. Hydrogen storage materials have a much greater energy storage capacity, which means that more energy can be stored in a smaller space compared to batteries and at a lower cost. This becomes particularly important when dealing with storing energy for transport applications such as cars and planes; a smaller sized system is much more practical.
Batteries also are characterized by a relatively short life span (<5 years), require a long period of time to charge (a few hours) and have negative environmental impacts upon disposal. Hydrogen storage requires no maintenance and has a much longer life span (> 20 years). In addition to being environmentally friendly, the storage systems contain no toxic chemicals.
The price of batteries also makes them impractical for large scale energy storage – for example, lithium-ion batteries cost about $2000/kWhr. Hydrogen is much cheaper than a battery, with a cost of 24¢/kWhr using water electrolysis. Even combined with the cost of a fuel cell, the cost of electrolysis hydrogen is approximately 48¢/kWhr, which is still less expensive than batteries.
The main response given when we surveyed people was that they think that it is simply too expensive to implement hydrogen as a major energy carrier. This is true – to an extent. Currently there is competition between traditional energy generation and new energy generation. The energy system is set up for fossil fuels and in order to change this, investment in infrastructure is required. But as stated above, eventually hydrogen energy will be a zero cost system and so these investments will eventually pay off!
Hydrogen can be used for energy applications now! The technology is developed enough to be able to use hydrogen for stationary applications and for small electronics. There are also demonstration or commercial models of cars, boats, and planes that have been developed by different groups around the world. We have developed a working hydrogen powered bike – we are simply improving it so that large-scale deployment is more viable.